About the DeFi-RDEB study
The DeFi-RDEB Phase 3 study is evaluating the safety and efficacy of FCX-007 (also known as D-Fi), an investigational gene therapy for the localized treatment of wounds that can lead to serious health complications in recessive dystrophic epidermolysis bullosa (RDEB).
What is FCX-007?
Castle Creek Biosciences is using its proprietary fibroblast technology platform to develop and evaluate FCX-007, which is also known as D-Fi. It is an innovative personalized treatment that is compatible with a patient's unique biology (because it uses the patient's own cells) and delivers functional Type VII collagen (COL7) protein where it is needed - at the site of wounds.
FCX-007 has been clinically studied in an earlier Phase 1/2 clinical study (NCT02810951), which assessed 6 patients with RDEB. In this study, 80% (8/10) of treated chronic wounds demonstrated complete wound healing 12 weeks after the first injection of FCX-007, while none of the untreated wounds were
healed. FCX-007 was well tolerated up to 52 weeks post-administration.
The distinctive therapeutic approach can be seamlessly integrated into a patient's current regimen. The COL7-corrected cells are delivered intradermally at the wounds in an outpatient setting in two or more treatment sessions, 12 weeks apart.
The FDA has granted Orphan Drug designation to FCX-007 for the treatment of Dystrophic Epidermolysis Bullosa, which includes RDEB. In addition, FCX-007 has been granted Rare Pediatric Disease designation, Fast Track designation and Regenerative Medicine Advanced Therapy (RMAT) designation by the FDA for treatment of RDEB.
Study Highlights
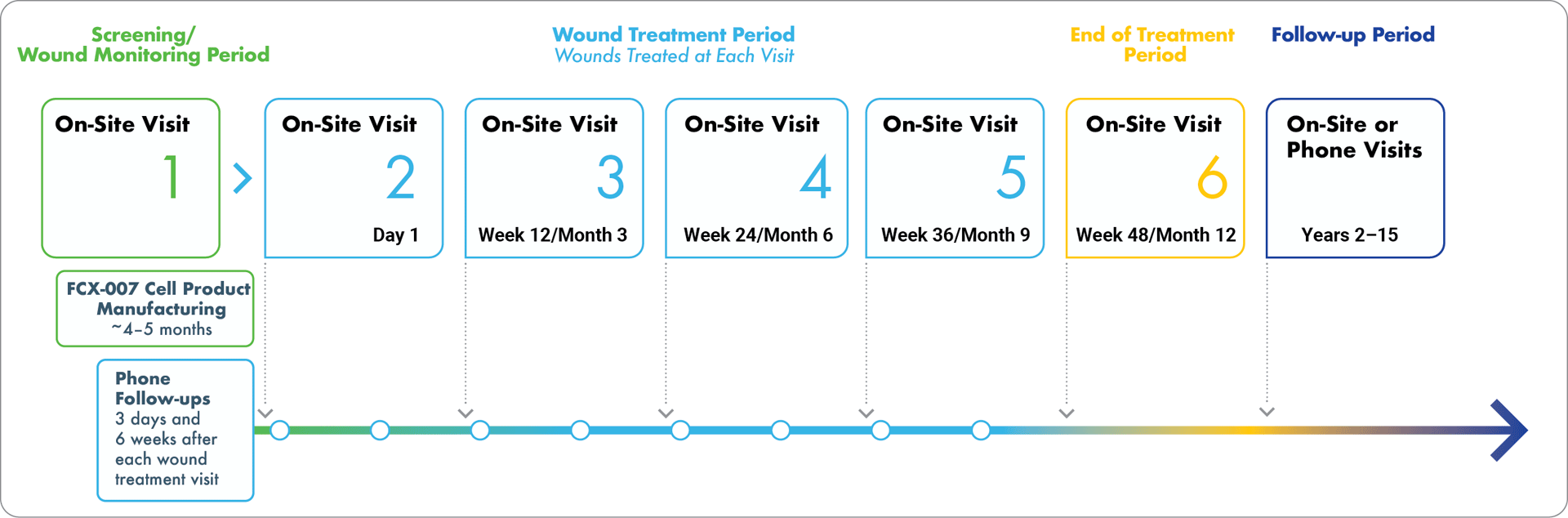
DeFi-RDEB is a multi-center, within-patient randomized, controlled, open-label, Phase 3 study of FCX-007 for the treatment of wounds in approximately 24 individuals living with RDEB. Up to three target wound pairs will be identified for each person. Each participant's target wounds will be randomly assigned to receive FCX-007 (treatment wound) or remain untreated (control wound). FCX-007 will be administered in two or more treatment sessions, with 12 weeks between treatment sessions. While this is not a surgical intervention, the use of a topical anesthetic or conscious sedation can be discussed with the study doctor if a patient is concerned about discomfort with
injection.
Wound closure and safety will be evaluated throughout the treatment period. Study participants who have received one or more injections of FCX-007 will be asked to participate in a long-term safety follow-up study (through 15 years).
All study-related costs, including investigational product, dermatologic exams and laboratory studies, and travel-related expenses will be covered for qualified participants. Remote evaluations are implemented when possible to protect the health and safety of study participants during the COVID-19 pandemic.
Eligibility Criteria
To participate in this study a patient needs to have a clinical diagnosis of RDEB with confirmation of COL7A1 genetic mutation. Other eligibility criteria include:
- Male or female ≥ 2 years of age at the screening visit.
- Participant must have at least two eligible wound sites identified.
