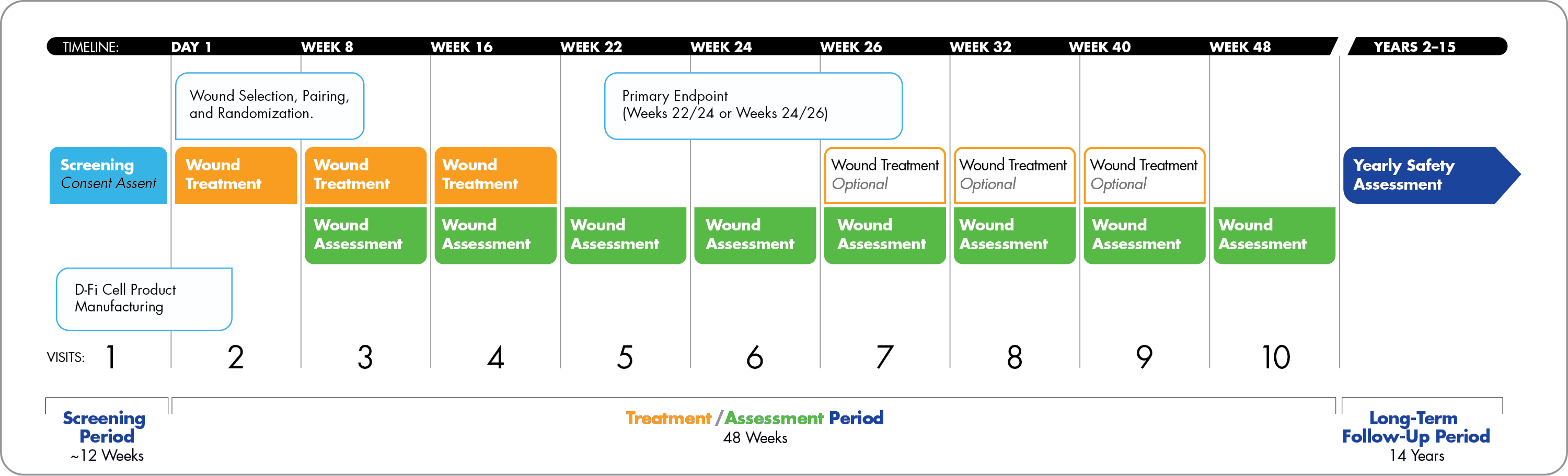
About the CCB-EB-304 study
The CCB-EB-304 Phase 3 study is evaluating the safety and efficacy of D-Fi, an investigational gene therapy for the localized treatment of wounds in dystrophic epidermolysis bullosa (DEB) that can lead to serious health complications.
What is D-Fi?
Castle Creek Biosciences is using its proprietary fibroblast technology platform to develop and evaluate D-Fi. It is an innovative personalized treatment that is compatible with a patient’s unique biology (because it uses the patient’s own cells) and delivers functional Type VII collagen (COL7) protein where it is needed – at the site of wounds.
The distinctive therapeutic approach can be seamlessly integrated into a patient’s current regimen. The COL7-corrected cells are delivered intradermally (by injection) at the wounds in an outpatient setting in three or more treatment sessions, 8 weeks apart.
The FDA has granted Orphan Drug designation to D-Fi for the treatment of Dystrophic Epidermolysis Bullosa (DEB). In addition, D-Fi has been granted Rare Pediatric Disease designation, Fast Track designation and Regenerative Medicine Advanced Therapy (RMAT) designation by the FDA.
Eligibility Criteria
To participate in this study a participant needs to have a clinical diagnosis of DEB with confirmation of COL7A1 genetic mutation. Other eligibility criteria include:
- Male or Female ≥ 18 months of age at the Screening visit.
- Participant must have at least two eligible wound sites identified.